Effective corrosion protection for steel pipes
Virtually any system of internal infrastructure and life support of residential buildings, municipal and commercial buildings or industrial facilities, by and large, is a developed network of pipelines connecting these or other objects of the system in a specific order.
In most cases, for example, when arranging a gas pipeline, hot and cold water supply, faecal or cable sewage systems, as well as heating and ventilation systems, underground, air or internal installation of metal pipes of various diameters and sizes is used.

Depending on the mode of operation and environmental conditions, metal pipes can be exposed to various adverse factors for a long time during operation. To solve this problem, the complex protection of pipelines against corrosion according to SNiP 2.03.11-85 “Protection of building structures against corrosion” was specially developed.
Methods of dealing with corrosion
To help the reader figure out how to ensure maximum pipeline durability, this article will look at some of the options for active and passive protection of metal products that make up pipeline engineering services.
Also here will be a detailed instruction, which details the basic principles of the implementation of corrosion protection for metal products intended for operation in aggressive conditions.

Classification of malicious factors
As mentioned above, the nature and degree of influence of external factors largely depends on the specific operating conditions, such as the location of the pipe, the chemical composition of the soil, the average annual temperature and the relative humidity of the environment, the presence of direct current sources nearby, etc.
According to the mechanism of occurrence and the degree of damaging effects, all harmful factors can be divided into several types.
- Atmospheric corrosion occurs when iron interacts with water vapor, which is contained in the ambient air, and also as a result of direct contact with water during precipitation. In the course of a chemical reaction, iron oxide is formed, or more simply, ordinary rust, which significantly reduces the strength of metal products, and over time can lead to their complete destruction.

- Chemical corrosion results from the interaction of iron with various active chemical compounds (acids, alkalis, etc.). At the same time, the proceeding chemical reactions lead to the formation of other compounds (salts, oxides, etc.), which, like rust, gradually destroy the metal.
- Electrochemical Corrosion occurs when the iron product is in an electrolyte medium for a long time (an aqueous solution of salts of different concentrations). At the same time, anodic and cathodic regions are formed on the surface of the metal, between which electric current flows. As a result of electrochemical emission, iron particles are transferred from one site to another, which leads to the destruction of a metal product.
- Exposure to negative temperatures when pipes are used to transport water, it causes it to freeze. When going into a solid state of aggregation, a crystal lattice forms in water, as a result of which its volume increases by 9%. Being in a closed space, the water begins to put pressure on the pipe walls, which ultimately leads to their rupture.

Note! A significant difference in average annual and average daily temperatures leads to significant fluctuations in the total length of the pipeline, which are caused by linear thermal expansion of the material. In order to prevent rupture of pipes and damage to supporting structures, thermal compensators must be installed at a certain distance on the line.
Soil analysis
In order to choose the most effective method of protection, it is necessary to have accurate information about the nature of the environment and the specific operating conditions of the steel pipeline. In the case of laying an internal or air line, this information can be obtained on the basis of subjective observations, as well as on the basis of the average annual climate regime for this region.
In the case of laying the underground pipeline, the corrosion resistance and durability of the metal is largely dependent on the physical parameters and chemical composition of the soil, so before digging a trench with your own hands, it is necessary to take soil samples for analysis to a specialized laboratory.

The most important indicators that need to be clarified in the process of analysis are the following soil qualities:
- The chemical composition and concentration of salts of various metals in groundwater. The density of the electrolyte and the electrical permeability of the soil largely depend on this indicator.
- Qualitative and quantitative indicators of soil acidity, which can cause both chemical oxidation and electrochemical corrosion of metal.
- Soil electrical resistance. The lower the value of electrical resistance, the more the metal is subject to destructive effects caused by electrochemical emission.

Tip! To obtain objective results of the analysis, soil samples must be removed from the soil layers in which the pipeline will pass.
Low temperature protection
In the case of underground or air laying of water supply and sewage networks, the most important condition for their uninterrupted operation is to protect the pipes from freezing and to maintain the water temperature at a level not lower than 0 ° С during the cold season.
To reduce the negative impact of the temperature factor of the environment, the following technical solutions are applied:
- Laying an underground pipeline at a depth exceeding the maximum depth of soil freezing for the region.
- Thermal insulation of air and underground lines using various materials with low thermal conductivity (mineral wool, foam segments, polypropylene sleeves).

- Backfilling of a pipeline trench with a low thermal conductivity bulk material (expanded clay, coal slag).
- Drainage of adjacent layers of soil in order to reduce its thermal conductivity.
- Laying of underground utilities in rigid closed boxes made of reinforced concrete, which provide an air gap between the pipe and the ground.
The most progressive method of how to protect pipes from freezing is to use a special casing consisting of a shell made of a heat-insulating material, inside of which an electrical heating element is laid.
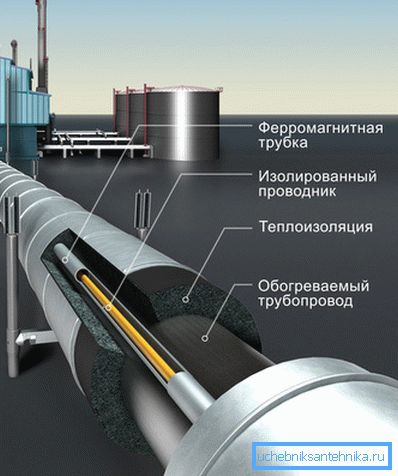
Note! The depth of soil freezing for each specific region, as well as the method of its calculation is governed by the normative documents SNiP 2.02.01-83 * "Foundations of buildings and structures" and SNiP 23-01-99 * Construction climatology.
External waterproofing coating
The most common way to combat metal corrosion is to apply a thin layer of durable waterproof protective material to its surface. The simplest example of an external protective coating is a conventional waterproof paint or enamel, for example, the protection of a gas pipe passing through the air is always carried out with the help of weather-resistant yellow enamel.
Underground plumbing and gas pipelines, as a rule, are assembled from pipes that are pre-coated on the outside with a thick layer of bitumen mastic and then wrapped with heavy technical paper. Coatings made of composite or polymeric materials are also highly effective.
Metal elements of sewage underground utilities from the inside and outside are covered with a thick layer of cement-sand mortar, which after solidification forms a uniform monolithic surface.

In order to independently select a suitable material for the outer coating, it is necessary to know that in order to ensure maximum protection it must simultaneously possess several qualities.
- After drying, the paint-and-lacquer coating should have a continuous homogeneous surface with high mechanical strength and absolute resistance to water.
- The protective film of waterproofing material, with the specified properties, must be elastic and not collapse under the influence of high or low temperatures.
- The starting material for the coating should have good fluidity, high covering ability, as well as good adhesion to the metal surface.
- Another indicator of a quality insulating material is that it must be an absolute dielectric. Due to this property, reliable protection of pipelines against stray currents, which increase the adverse effects of electrochemical corrosion, is provided.

Tip! The most effective solutions for isolating metal from the environment are considered to be compositions based on bitumen resins, two-component polymeric compositions, as well as rolled polymeric materials on a self-adhesive basis.
Active and passive electrochemical protection
Underground utilities are more susceptible to the onset of corrosion than air and internal pipelines, because they are constantly in the electrolyte medium, which is a solution of salts contained in the composition of groundwater.
In order to minimize the destructive effect caused by the reaction of iron with a water-salt electrolyte solution, active and passive methods of electrochemical protection are used.
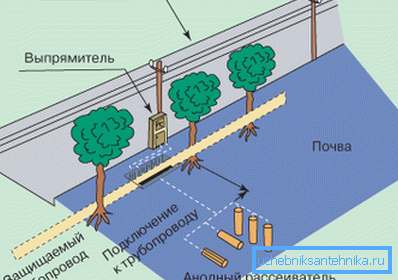
- Active cathodic method consists in the directional movement of electrons in a chain of direct electric current. For its implementation, a pipeline is connected to the negative pole of the DC source, and an anode grounding rod is connected to the positive pole, which is buried in the ground nearby. After energization, the electrical circuit closes through the soil electrolyte, as a result of which free electrons begin to move from the ground rod to the pipeline. Thus, the grounding electrode gradually collapses, and the released electrons instead of the pipeline react with the electrolyte.
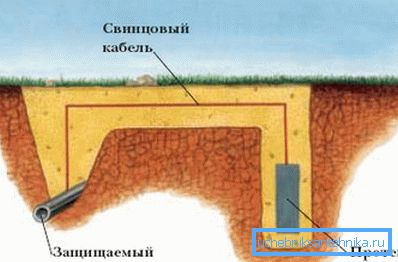
- Passive sacrificial protection of pipelines is that, next to iron, an electrode of a more electronegative metal, such as zinc or magnesium, is placed in the ground and electrically connected to each other through a controlled load. In the electrolyte medium, they form a galvanic couple, which in the course of the reaction, as in the previous case, causes the movement of electrons from the zinc protector to the protected pipeline.
- Electrical drainage protection It is also a passive method, which is performed by connecting the pipeline to the grounding circuit, made in accordance with the EMP. This method helps to get rid of the occurrence of stray currents and is used in the case of the location of the pipeline near the contact electrical network of land or rail transport.
Note! A good example of a passive protector protection is the well-known zinc coating of iron products, or more simply, zinc plating.
Conclusion
Each of the above methods has its advantages and disadvantages, so they should be used depending on the specific conditions that have arisen. In conclusion, it should be said that, regardless of the chosen method, the cost of repair and replacement of the pipeline will cost much more than the cost of the most complex and time-consuming protection.
For more information, you can watch the video in this article or read similar materials on our website.