How to get rid of iron in the well
The drilled wells for water are saturated with iron, exceeding the norm according to GOST “Drinking Waters” - 0.3 mg / l. At the same time, water acquires a yellowish color, the taste of iron, and compounds and iron bacteria are deposited on the walls of pipelines and reduce the diameter. Then the consumer gets rid of iron in the well - it has a detrimental effect on human health and leaves stains on clothes after washing.
In water, iron is present in the bivalent form Fe2 +, its taste and smell is felt, but at the same time the water will be clear. After oxidizing, iron passes into the trivalent form Fe3 + and falls out in the form of red flakes of iron hydroxide, many methods of deferrization are based on this principle.
The removal of iron from water is achieved by non-reagent methods, which include oxidation and filtration, but in some cases it is impossible to do without additional chemical treatment of water.
Oxidation methods

Three oxidation methods are common:
- Simple aeration - iron is oxidized by air. Water is sprayed, or using injectors, aeration. This method is effective in cases where the concentration of Fe2 + does not exceed 10 mg / l, pH is not less than 6.8, and the hydrogen sulfide content is not more than 2 mg / l.
- Chemical oxidants change the pH and oxidize organic compounds that prevent the transfer of iron to Fe3 +. Sodium hypochlorite or chlorine is used as an oxidizing agent, less frequently ozone and potassium permanganate. Disadvantages are the formation of carcinogenic intermediates.
- For catalytic oxidation, materials that remove Fe2 +, manganese and hydrogen sulfide are used. Catalytic material - Birm, Pyrolox, Centaur. Materials used in modern installations deferrization.
Purification by Filtration

Method of removing Fe2 + and Fe3 + by filtration. Water in which iron and oxygen is dissolved passes through the granular charge layer and releases iron on the surface of the grains. This forms a catalytic film, its composition is iron hydroxide. This film accelerates oxidation. The filter material is quartz sand, zeolite, polystyrene foam.
Cleaning scheme: aerator, filter and tank for collecting clean water. From the well, water is supplied to the aerator for oxidizing iron, after that - a quick filter, where cleaning goes from top to bottom and in the opposite direction.
The water that enters the purification must contain 0.6-0.9 mg of oxygen per 1 mg of Fe2 + and have a pH of at least 6.8.
With a low pH, as well as with a high concentration of organic compounds, the deferrization by simple aeration is extremely inefficient.
In order to raise the pH, it is necessary to alkalize the water with the help of dolomite crumb, through which the purified water is passed, or lime. The second method is ineffective if organic compounds are present in the groundwater - in this case it is impossible to do without treatment with an oxidizing agent.
Groundwater from wells drilled in the floodplains of rivers contain a high concentration of organic compounds and humic acids; this should be taken into account when choosing a drilling site.
In the process of filtering iron compounds accumulate in the pores of the filter, which leads to a decrease in its throughput. To restore this function, it is necessary to rinse with reverse flow of purified water.
Water deironing with catalytic loading
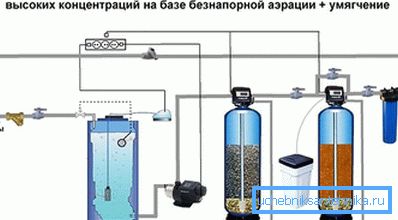
This method of cleaning is carried out thanks to the filters, which are simultaneously oxidizing agents and filtering medium for the retention not only of iron, manganese, but also of hydrogen sulfide. When water comes in contact with such a load, Fe2 + and Fe3 + are transferred from the dissolved form to the undissolved form and are retained on the surface of the loading grains. Filter materials with catalytic properties are used as loading:
- Birm, a filtering material based on aluminosilicate with manganese dioxide, is used to purify groundwater with an iron concentration up to 7 mg / l and manganese below 0.5 mg / l, very afraid of chlorine, regeneration occurs by reverse flow of water.
- Pyrolox is a catalytic material of natural origin based on manganese ore that allows water purification with an iron concentration of up to 20 mg / l for manganese 5 mg / l. Additional oxidation by chlorination is allowed to increase the cleaning efficiency. Regeneration is performed by backwashing, but due to the fact that Pyrolox has a high bulk density, a large amount of water is needed for flushing.
Filters that use these backfills can solve a complex task of cleaning, but along with their merits they have some disadvantages:
- high cost of loading;
- short term operation, after which you need to change the load.
The choice of one or another type of load depends on the chemical composition of water and its estimated consumption.
Purification with ion exchange resins

Purification using ion exchange must be carried out separately, since this type of loading solves a wide range of tasks. In addition to purification from iron, manganese and organic compounds, the hardness salts are removed. But the regeneration of such a load can no longer be performed by backwashing the water; here a salt-based regenerating solution is used, as a rule, it is NaCl, which increases the operating costs of the installation.
Filters based on ion exchange resins do a good job of cleaning up dissolved Fe2 +, but Fe3 + quickly clogs up.

The choice of a particular purification scheme is done after thoroughly chemical analysis of water and calculation of water consumption. Improperly selected technology can lead to failure of expensive loading or not cope with its task.
Video
If you are interested in the opinion of a professional about which type of water treatment is the most effective, then you will be interested in the video we offer to watch: